How ozone works
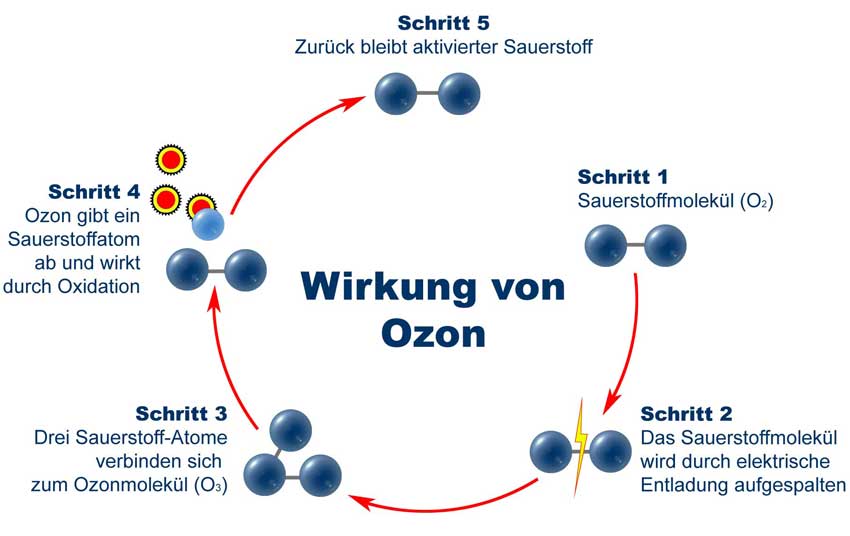
Ozone is a special form of oxygen. Normally, two oxygen atoms combine to an oxygen molecule. The ozone molecule on the other hand consists of a loose connection of three oxygen atoms. If this molecule disintegrates, the individual atoms are looking for new reactant. Influences exerted by ozone on the biochemical reaction process, based on the strong oxidizing effect. Ozone is the strongest technically available oxidant and efficient disinfectant at all. At the same time it is the most environmentally friendly means, because it consists of only three atoms of oxygen and carries no other chemical into the process.
Oxidation smells of smokers, animals, waste water, ammonia and fire odors are removed. Ozone removes odors by, for example, hydrogen sulfide destroys the molecules that are responsible for the odors (H2S), ammonia and other organic compounds by chemical reactions. All germs, bacteria and fungi are killed by ozone.
Why ozone generators?
Ozone can not be stored and decays with a half-life of 20 minute oxygen. Therefore, it can not be bought like other manufactured gases used in pressurized cylinders. Prior to its application, it must be generated by electric discharge in place by ozone generators of oxygen / nitrogen mixtures. Our devices generate ozone from the contained in the normal atmospheric oxygen. Therefore, no further supplies are needed and make the device easy to use. All you need is a standard socket for electricity.

